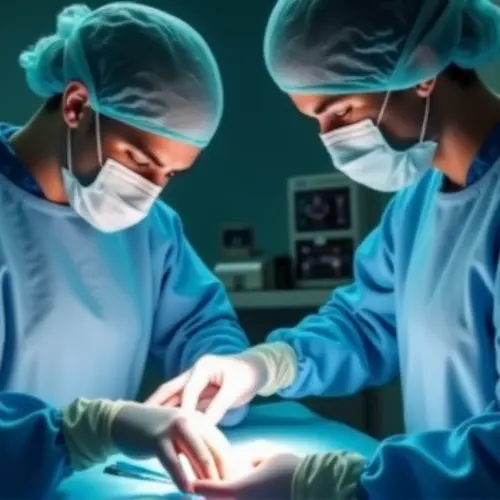
Revolutionary Treatment Restores Vision
European regulators have approved a groundbreaking gene therapy that restores vision in patients with rare retinal diseases. This landmark decision comes after successful clinical trials demonstrated significant vision improvement in patients with inherited retinal dystrophies.
How the Therapy Works
The treatment uses modified adeno-associated viruses (AAVs) to deliver functional copies of defective genes directly to retinal cells. By replacing the mutated RPE65 gene responsible for conditions like Leber congenital amaurosis (LCA), the therapy enables the production of essential light-sensitive proteins. Unlike conventional treatments, this single-dose therapy shows long-lasting effects with patients maintaining improved vision for over three years.
Clinical Trial Results
In recent trials:
- 11 children born blind gained measurable visual acuity
- 90% of patients reduced dependency on supplemental treatments
- 68-85% became completely treatment-free after therapy
Patients reported life-changing improvements in daily activities, social integration, and overall quality of life.
Regulatory Milestone
The European Medicines Agency's approval follows similar authorizations in the US and UK. This therapy represents the first approved treatment for several rare retinal conditions that previously had no available treatments. Pharmaceutical companies are now working with European healthcare systems to establish innovative payment models addressing the therapy's €345,000 per eye cost.

 Nederlands
Nederlands
 English
English
 French
French
 Deutsch
Deutsch
 Espaniol
Espaniol
 Portugese
Portugese









